Introduction: Women with bleeding disorders experience bleeding in mucocutaneous tissues, uterine bleeding, and in muscles and joints. In men, bleeding of more than 1 or 2 bleeds per year is justification for starting prophylaxis with factor and non-factor replacement therapy. At Banner MD Anderson Cancer Center (BMDACC), women are offered prophylaxis according to the same standard. Uterine bleeding classified as excessive or disruptive to daily activity is considered bleeding requiring prophylaxis.
Aim: Describe the number of women in the bleeding disorder database from June 2018 to December 2022 with Hemophilia A (HemA), Hemophilia B (HemB) and Von Willebrand Disease (VWD) with respect to number on prophylaxis to prevent bleeds. Specific factor levels and subtype of VWD were not gathered as part of this project. Rather, patients were offered prophylaxis based on symptoms of bleeding. Prophylaxis is defined as treatment to prevent bleeding associated with day-to-day activity, but not prior to planned procedures. The type of treatment was recorded, type of access if needed (peripheral intravenous access v. central venous access) and why treatment was started.
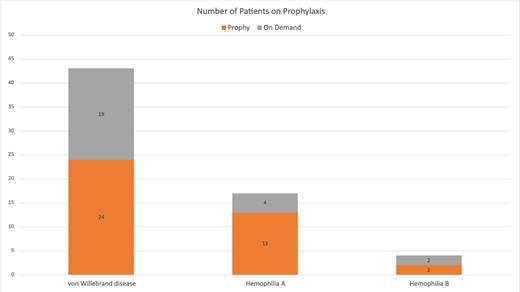
Results: Among 101 female patients in the BMDACC bleeding disorder database, 64 had a diagnosis of HemA, HemB or VWD. Among HemA, 13 of 17 (76%) were on prophylaxis with factor replacement or emicizumab. Joint bleeding and pain were the most common reasons for starting prophylaxis in 6 of 13 patients and menorrhagia was the second most common in 3 patients. Among HemB, 2 of 4 (50%) were on factor replacement. Rectal bleeding and menorrhagia were reasons to start prophylaxis. Among VWD, 24 of 43 (56%) were on prophylaxis with one or more of the following treatments: factor replacement, desmopressin or antifibrinolytic replacement. Menorrhagia was the most common reason for starting prophylaxis in 13 of 24 patients and joint bleeding was the next most common in 4 patients. Additional reasons were trauma, mucocutaneous, urinary tract bleeding, and bleeding during pregnancy.
Patients with VWD were much more likely to require central IV access in the form of a chest port-a-cath 14 of 43 (33%) than patients with HemA or HemB, of which no patients required central access. Patients with HemA or HemB were more likely to use factor replacement than VWD to prevent bleeding symptoms.
Patients with VWD were more likely than patients with HemA or Hem B to have a diagnosis prior to age 18; VWD had 16 of 43 (37%), HemA had 3 of 17 (18%) and HemB had 0 of 4 (0%).
Conclusions: Female patients with inherited bleeding disorders have bleeding symptoms that may require the initiation of routine treatment to prevent bleeding and should be offered treatment with hemostatic agents to prevent bleeding when bleeding frequency exceeds more than 1 or 2 bleeds per year. Heavy menstrual bleeding, joint bleeding, joint pain, gastrointestinal and genitourinary bleeding are reported in this patient population as reasons to start prophylaxis with hemostatic agents. Further study is underway to assess the efficacy of starting prophylaxis in these patients. In the Author's experience, once factor replacement is started, patients are pleased with the change in their health status after initiation of treatment and continue to be compliant with treatment recommendations although they may have difficulty in administering those treatments. Patients with VWD were much more likely than those with HemA or HemB to require placement of a central venous access device. Further study into this observation is needed to understand why this is so.
Disclosures
Nance:Sanofi: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Alnylam: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal